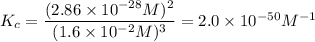Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript...

Chemistry, 26.02.2020 20:55 jessicajamah3289
Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript 3 (g).
At 298 K, the equilibrium concentration of O2 is 1.6 x 10-2 M, and the equilibrium concentration of O3 is 2.86 x 10-28 M. What is the equilibrium constant of the reaction at this temperature?
A) 2.0 x 10^-10
B) 2.0 x 10^10
C) 1.8 x 10^-10
D) 1.8 x 10^10

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Questions

Mathematics, 22.09.2021 19:50

Chemistry, 22.09.2021 19:50

Mathematics, 22.09.2021 19:50

Mathematics, 22.09.2021 19:50



English, 22.09.2021 19:50

History, 22.09.2021 19:50

Mathematics, 22.09.2021 19:50

Mathematics, 22.09.2021 19:50




Physics, 22.09.2021 19:50


Mathematics, 22.09.2021 19:50


Mathematics, 22.09.2021 19:50

Mathematics, 22.09.2021 19:50


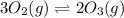
![K_c=\dfrac{[O_3g)]^2}{[O_2(g)]^3}](/tpl/images/0525/4129/a4203.png)