
A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the copper half-cell, Cu2+ (aq, 1.00 M) | Cu (s). The hydrogen gas pressure is 1.00 bar. A value of 0.490 V is recorded for E Cell at 298 K. Determine the concentration of H+ and the pH of the solution.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the co...
Questions


English, 18.11.2020 22:00

Health, 18.11.2020 22:00


Mathematics, 18.11.2020 22:00


Mathematics, 18.11.2020 22:00


Mathematics, 18.11.2020 22:00

Arts, 18.11.2020 22:00



English, 18.11.2020 22:00

Mathematics, 18.11.2020 22:00

English, 18.11.2020 22:00

English, 18.11.2020 22:00

English, 18.11.2020 22:00

Biology, 18.11.2020 22:00


Mathematics, 18.11.2020 22:00

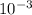 M and the pH = 2.6 of the solution
M and the pH = 2.6 of the solution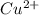 ) is the cathode and hydrogen (
) is the cathode and hydrogen (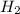 ) is the anode.
) is the anode.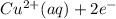 ⇒ Cu(s)
⇒ Cu(s)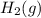 ⇒
⇒ 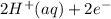
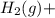
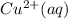 ⇒
⇒ 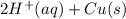
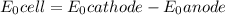
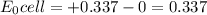
![\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/22043.png)
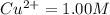 but
but 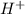 is unknown. we solve this using hernst equation.
is unknown. we solve this using hernst equation.![E = E^{0} -\frac{0.0257}{n}ln\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/8e268.png)
![0.490 = 0.337 -\frac{0.0257}{2}ln\frac{[H^{+}]^{2} }{[1][1]}](/tpl/images/0525/6315/43505.png)
![ln{[H^{+}]^{2} } = -11.9](/tpl/images/0525/6315/8c81e.png)
![2ln{[H^{+}] } = -11.9](/tpl/images/0525/6315/31a40.png)
![ln{[H^{+}] } = -5.95](/tpl/images/0525/6315/e2ee5.png)
![[H^{+}] = 3* 10^{-3} M](/tpl/images/0525/6315/d9bb9.png)


