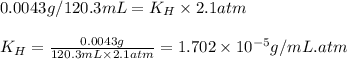Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
If 120.3 mL of water is shaken with oxygen gas at 2.1 atm, it will dissolve 0.0043 g O2. Estimate th...
Questions

Mathematics, 21.09.2019 20:30




Computers and Technology, 21.09.2019 20:30

Mathematics, 21.09.2019 20:30


English, 21.09.2019 20:30

Mathematics, 21.09.2019 20:30

Physics, 21.09.2019 20:30

History, 21.09.2019 20:30

History, 21.09.2019 20:30

Business, 21.09.2019 20:30

Social Studies, 21.09.2019 20:30


Business, 21.09.2019 20:30

Mathematics, 21.09.2019 20:30

Mathematics, 21.09.2019 20:30


Social Studies, 21.09.2019 20:30

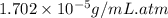
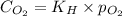
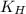 = Henry's constant = ?
= Henry's constant = ?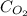 = solubility of oxygen gas =
= solubility of oxygen gas = 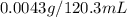
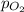 = partial pressure of oxygen gas = 2.1 atm
= partial pressure of oxygen gas = 2.1 atm