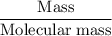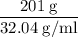Chemistry, 26.02.2020 23:21 annalaurie7
Methanol (CH3OH) burns in air according to the equation 2CH3OH + 3O2 → 2CO2 + 4H2O If 201 g of methanol are used up in a combustion process, what is the mass of H2O produced?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Methanol (CH3OH) burns in air according to the equation 2CH3OH + 3O2 → 2CO2 + 4H2O If 201 g of metha...
Questions



Mathematics, 17.12.2020 16:50



History, 17.12.2020 16:50



Mathematics, 17.12.2020 16:50


Mathematics, 17.12.2020 16:50

Spanish, 17.12.2020 16:50

Mathematics, 17.12.2020 16:50

Mathematics, 17.12.2020 16:50


History, 17.12.2020 16:50



History, 17.12.2020 16:50