

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
The rate constant of a particular first order reaction is 5.45 x 10^-2 sec^-1 at 40.0 oC. What is th...
Questions

Mathematics, 26.04.2021 20:50

Mathematics, 26.04.2021 20:50

Mathematics, 26.04.2021 20:50

Mathematics, 26.04.2021 20:50

Mathematics, 26.04.2021 20:50




Mathematics, 26.04.2021 20:50

Mathematics, 26.04.2021 20:50

Mathematics, 26.04.2021 20:50



Computers and Technology, 26.04.2021 20:50

English, 26.04.2021 20:50


Mathematics, 26.04.2021 20:50



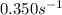
![\ln(\frac{K_{65^oC}}{K_{40^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0525/7564/05a35.png)
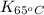 = equilibrium constant at 65°C = ?
= equilibrium constant at 65°C = ?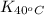 = equilibrium constant at 40°C =
= equilibrium constant at 40°C = 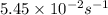
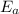 = Activation energy of the reaction = 65.5 kJ/mol = 65500 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy of the reaction = 65.5 kJ/mol = 65500 J/mol (Conversion factor: 1 kJ = 1000 J)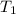 = initial temperature =
= initial temperature = ![40^oC=[40+273]K=313K](/tpl/images/0525/7564/84088.png)
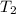 = final temperature =
= final temperature = ![65^oC=[65+273]K=338K](/tpl/images/0525/7564/eb1a6.png)
![\ln(\frac{K_{65^oC}}{5.45\times 10^{-2}})=\frac{65500J/mol}{8.314J/mol.K}[\frac{1}{313}-\frac{1}{338}]\\\\K_{65^oC}=0.350s^{-1}](/tpl/images/0525/7564/e250a.png)


