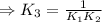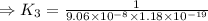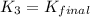Chemistry, 27.02.2020 00:10 barnhill6534
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equilibrium constant Kfinal for the following reaction? S2−(aq)+2H+(aq)⇌H2S(aq)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 14:20
Compounds a and b react to form compounds c and d according to the equation: aa + bb → cc + dd. under which conditions will the rate law be given by the equation: rate = k[a]a[b]b? a. the reaction takes place in one step. b. the reaction is endothermic. c. the reaction is exothermic. d. the reaction involves more than one step.
Answers: 3
You know the right answer?
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equ...
Questions


Spanish, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01

History, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01


Arts, 18.10.2020 16:01

History, 18.10.2020 16:01



Mathematics, 18.10.2020 16:01






Mathematics, 18.10.2020 16:01

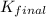 is 9.35× 10²⁵
is 9.35× 10²⁵![k=\frac{[C]^z}{[A]^x[B]^y}](/tpl/images/0525/8601/0a5aa.png)
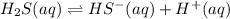
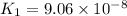
![K_1=\frac{[HS^-][H^+]}{[H_2S]}](/tpl/images/0525/8601/e1e1d.png)
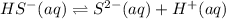
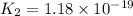
![K_2=\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/661de.png)
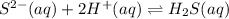
![K_3=\frac{[H_2S]}{[S^{2-}][H^+]^2}](/tpl/images/0525/8601/58537.png)
![k_1k_2=\frac{[HS^-][H^+]}{[H_2S]}\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/53894.png)
![\Rightarrow k_1k_2=\frac{[S^{2-}][H^+]^2}{[H_2S]}](/tpl/images/0525/8601/762f9.png)