Chemistry, 27.02.2020 00:31 dakotakeating4513
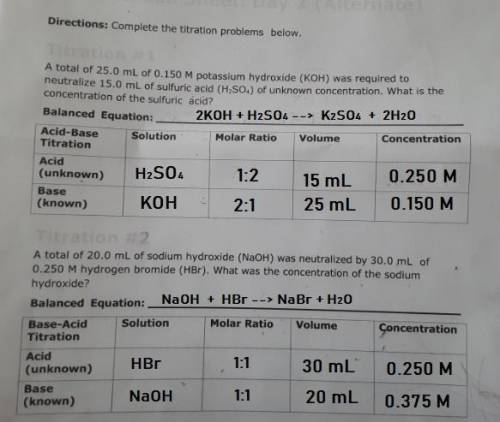
PLEASE HELP! A total of 25.0 mL of 0.150 M potassium hydroxide (KOH) was required to neutralize 15.0 mL of sulfuric acid (H2SO4) of unknown concentration. What is the concentration of the sulfuric acid?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1


Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

Chemistry, 23.06.2019 12:30
The equilibrium constant kc for the reaction 2 nocl(g) → 2 no(g) + cl2(g) is 0.453 at a certain temperature. a mixture of nocl, no, and cl2 with concentrations 1.30, 1.20, and 0.600 m, respectively, was introduced into a container at this temperature. which of the following is true? 1. no apparent reaction takes place. 2. [cl2] = 0.30 m at equilibrium. 3. nocl(g) is produced until equilibrium is reached. 4. [nocl] = [no] = [cl2] at equilibrium. 5. cl2(g) is produced until equilibrium is
Answers: 3
You know the right answer?
PLEASE HELP! A total of 25.0 mL of 0.150 M potassium hydroxide (KOH) was required to neutralize 15.0...
Questions

Social Studies, 21.09.2019 01:40


Mathematics, 21.09.2019 01:50

Mathematics, 21.09.2019 01:50

Chemistry, 21.09.2019 01:50

Physics, 21.09.2019 01:50

Mathematics, 21.09.2019 01:50

Mathematics, 21.09.2019 01:50


Chemistry, 21.09.2019 01:50

Biology, 21.09.2019 01:50

Mathematics, 21.09.2019 01:50

History, 21.09.2019 01:50

Mathematics, 21.09.2019 01:50

Mathematics, 21.09.2019 01:50