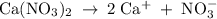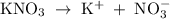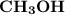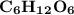Chemistry, 27.02.2020 00:57 delaneynagle3368
Which of the following aqueous solutions has the highest boiling point?A. 1.25 M Ca(NO3)2B. 1.25 M KNO3C. 1.25 M CH3OHD. 2.50 M C6H12O6E. None of the above (all have the same boiling point)

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
Which of the following aqueous solutions has the highest boiling point?A. 1.25 M Ca(NO3)2B. 1.25 M K...
Questions


Mathematics, 08.10.2019 21:00

Mathematics, 08.10.2019 21:00


History, 08.10.2019 21:00

Spanish, 08.10.2019 21:00


Chemistry, 08.10.2019 21:00


Mathematics, 08.10.2019 21:00


Health, 08.10.2019 21:00

Biology, 08.10.2019 21:00


Mathematics, 08.10.2019 21:00

Mathematics, 08.10.2019 21:00

History, 08.10.2019 21:00

Geography, 08.10.2019 21:00

Biology, 08.10.2019 21:00

History, 08.10.2019 21:00

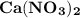 . Thus, option A is correct.
. Thus, option A is correct.