
Chemistry, 27.02.2020 01:24 realpcy7515
The student is now told that the four solids, in no particular order, are aluminum chloride (AlCl3AlCl3), sugar (C6H12O6C6H12O6), benzoic acid (C6H5COOHC6H5COOH), and sodium bromide (NaBrNaBr). Assuming that conductivity is correlated to the number of ions in solution, rank the four substances based on how well a 0.20 MM solution in water will conduct electricity.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
You know the right answer?
The student is now told that the four solids, in no particular order, are aluminum chloride (AlCl3Al...
Questions

Arts, 06.02.2021 02:50




History, 06.02.2021 02:50

Biology, 06.02.2021 02:50


Physics, 06.02.2021 02:50

Mathematics, 06.02.2021 02:50



Mathematics, 06.02.2021 02:50

English, 06.02.2021 02:50


Mathematics, 06.02.2021 02:50


Mathematics, 06.02.2021 02:50


Mathematics, 06.02.2021 02:50

Mathematics, 06.02.2021 02:50

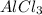 > NaBr > Benzoic acid > sugar
> NaBr > Benzoic acid > sugar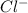 ions and thus, produces 4 ions.
ions and thus, produces 4 ions.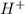 and
and 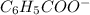 .
.

