
Chemistry, 27.02.2020 01:54 psychocatgirl1
Consider the reaction of ethyl acetate with sodium hydroxide: CH3COOC2H5(aq)+NaOH(aq)⇌CH3COONa(aq )+C2H5OH(aq) The reaction is first order in NaOH and second order overall. What is the rate law? View Available Hint(s) Consider the reaction of ethyl acetate with sodium hydroxide: The reaction is first order in and second order overall. What is the rate law? rate=k[CH3COOC2H5]2[NaOH]2 rate=k[CH3COOC2H5][NaOH]2 rate=k[NaOH] rate=k[CH3COOC2H5] rate=k[CH3COOC2H5][NaOH] rate=k[CH3COOC2H5]2[NaOH]

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Consider the reaction of ethyl acetate with sodium hydroxide: CH3COOC2H5(aq)+NaOH(aq)⇌CH3COONa(aq )+...
Questions

History, 04.11.2020 21:20

Health, 04.11.2020 21:20



Mathematics, 04.11.2020 21:20


Biology, 04.11.2020 21:20





Mathematics, 04.11.2020 21:20

English, 04.11.2020 21:20


Mathematics, 04.11.2020 21:20

Biology, 04.11.2020 21:20


Engineering, 04.11.2020 21:20


![rate=k[CH_3COOC_2H_5]^1[NaOH]^1](/tpl/images/0526/0774/1e7fe.png)
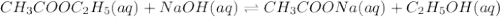
![rate=k[CH_3COOC_2H_5]^x[NaOH]^y](/tpl/images/0526/0774/4cba9.png)
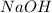
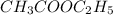 is 1 and rate law is :
is 1 and rate law is : 

