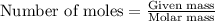Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 Pb(ClO3)2 with aqueous NaI . NaI. Include phases. chemical equation: What mass of precipitate will form if 1.50 L 1.50 L of highly concentrated Pb ( ClO 3 ) 2 Pb(ClO3)2 is mixed with 0.400 L 0.130 M NaI 0.400 L 0.130 M NaI ? Assume the reaction goes to completion

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 Pb(ClO3)2 with aqueous NaI ....
Questions





Biology, 26.08.2019 01:00

Mathematics, 26.08.2019 01:00


History, 26.08.2019 01:00

Health, 26.08.2019 01:00



Advanced Placement (AP), 26.08.2019 01:00


Mathematics, 26.08.2019 01:00

History, 26.08.2019 01:00


History, 26.08.2019 01:00

History, 26.08.2019 01:00

Biology, 26.08.2019 01:00

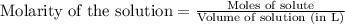
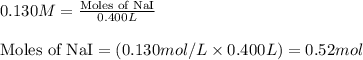
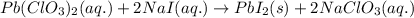
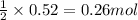 of lead (II) iodide
of lead (II) iodide