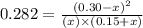Chemistry, 27.02.2020 02:01 FreyaLouise
He following reaction becomes possible: H2gI2g 2HIg The equilibrium constant K for this reaction is 0.282 at the temperature of the flask. Calculate the equilibrium molarity of H2. Round your answer to two decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

You know the right answer?
He following reaction becomes possible: H2gI2g 2HIg The equilibrium constant K for this reaction is...
Questions



Computers and Technology, 18.11.2019 20:31


Chemistry, 18.11.2019 20:31

Mathematics, 18.11.2019 20:31










Social Studies, 18.11.2019 20:31


Physics, 18.11.2019 20:31

Mathematics, 18.11.2019 20:31





![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0526/1028/8a740.png)