
Chemistry, 27.02.2020 02:02 TombRaider167
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid serious problems. One such breathing mixture contains helium, oxygen, and carbon dioxide. Determine the partial pressure of oxygen when the total pressure in the tank is 201.4 kPa if PHe = 125.4 kPa and PCO2= 18.2 kPa? Must show all work that leads to answer for credit

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1


Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3
You know the right answer?
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid s...
Questions

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

English, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Biology, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Health, 13.09.2020 03:01

Social Studies, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

Mathematics, 13.09.2020 03:01

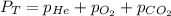
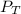 = 201.4 kPa
= 201.4 kPa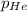 = 125.4 kPa
= 125.4 kPa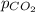 = 18.2 kPa
= 18.2 kPa![201.4=125.4+p_{O_2}+18.2\\\\p_{O_2}=201.4-[125.4+18.2]=57.8kPa](/tpl/images/0526/1098/fa869.png)


