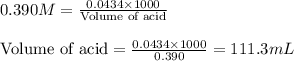Chemistry, 27.02.2020 02:21 jaidalynkimora
Suppose that you have 115 mL of a buffer that is 0.460 M in both benzoic acid ( C 6 H 5 COOH ) and its conjugate base ( C 6 H 5 COO − ) . Calculate the maximum volume of 0.390 M HCl that can be added to the buffer before its buffering capacity is lost.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Suppose that you have 115 mL of a buffer that is 0.460 M in both benzoic acid ( C 6 H 5 COOH ) and i...
Questions


Mathematics, 15.07.2019 10:30



Chemistry, 15.07.2019 10:30

History, 15.07.2019 10:30



History, 15.07.2019 10:30

Chemistry, 15.07.2019 10:30




Mathematics, 15.07.2019 10:30

Geography, 15.07.2019 10:30

Spanish, 15.07.2019 10:30

Mathematics, 15.07.2019 10:30

Social Studies, 15.07.2019 10:30

Mathematics, 15.07.2019 10:30

![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0526/1364/e4eea.png)
![pH=pK_a+\log(\frac{[C_6H_5COO^-]}{[C_6H_5COOH]})](/tpl/images/0526/1364/e41e3.png) .....(1)
.....(1)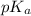 = negative logarithm of acid dissociation constant of benzoic acid = 4.2
= negative logarithm of acid dissociation constant of benzoic acid = 4.2![[C_6H_5COOH]=0.460M](/tpl/images/0526/1364/fc044.png)
![[C_6H_5COO^-]=0.460M](/tpl/images/0526/1364/ef922.png)
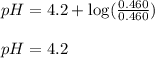
![3.2=4.2+\log(\frac{[C_6H_5COO^-]}{[C_6H_5COOH]})\\\\\frac{[C_6H_5COO^-]}{[C_6H_5COOH]}=0.1](/tpl/images/0526/1364/89428.png)
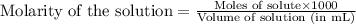 ......(2)
......(2)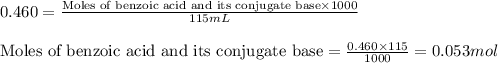
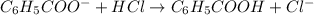
![\frac{[C_6H_5COO^-]-x}{[C_6H_5COOH]+x}=0.1\\\\\frac{0.053-x}{0.053+x}=0.1\\\\x=0.0434](/tpl/images/0526/1364/881c9.png)