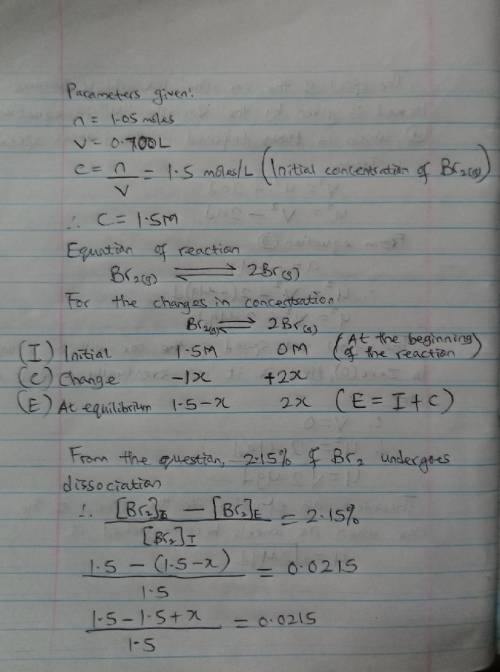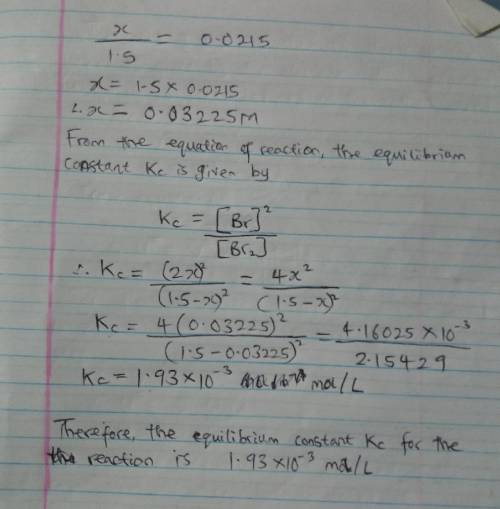Chemistry, 27.02.2020 03:11 greatsavagebeast
When 1.05 moles of Br2 are put in a 0.700−L flask, 2.15 percent of the Br2 undergoes dissociation. Calculate the equilibrium constant Kc for the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


You know the right answer?
When 1.05 moles of Br2 are put in a 0.700−L flask, 2.15 percent of the Br2 undergoes dissociation. C...
Questions

History, 17.07.2020 01:01

History, 17.07.2020 01:01



Mathematics, 17.07.2020 01:01


Mathematics, 17.07.2020 01:01


Mathematics, 17.07.2020 01:01






Chemistry, 17.07.2020 01:01

Law, 17.07.2020 01:01


Mathematics, 17.07.2020 01:01

History, 17.07.2020 01:01