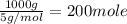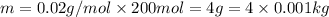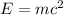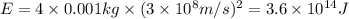Chemistry, 27.02.2020 04:52 nell1234565
The loss of mass in nuclear fusion is 0.02 g per mole of fuel. The molecular weight of the fuel is 5 g/mole. The amount of energy produced by consuming 1 kg of fuel in nuclear fusion is kWh.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
The loss of mass in nuclear fusion is 0.02 g per mole of fuel. The molecular weight of the fuel is 5...
Questions

History, 24.09.2019 06:00



History, 24.09.2019 06:00

Business, 24.09.2019 06:00



History, 24.09.2019 06:00

Physics, 24.09.2019 06:00

Mathematics, 24.09.2019 06:00

Mathematics, 24.09.2019 06:00



French, 24.09.2019 06:00

Physics, 24.09.2019 06:00

Physics, 24.09.2019 06:00

Health, 24.09.2019 06:00

Health, 24.09.2019 06:00


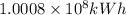 kWh.
kWh.