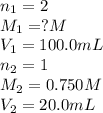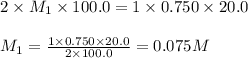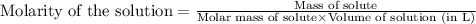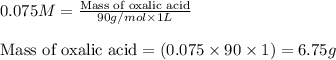Chemistry, 27.02.2020 05:47 utjfkdndidndldn62121
A sample of oxalic acid (a diprotic acid of the formula H2C2O4) is dissolved in enough water to make 1.00 L of solution. A 100.0 mL sample of this solution is titrated with a solution of sodium hydroxide of concentration 0.750 M and requires 20.0 mL of sodium hydroxide to reach the end point. Calculate the mass of the original oxalic acid sample.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
A sample of oxalic acid (a diprotic acid of the formula H2C2O4) is dissolved in enough water to make...
Questions

Mathematics, 20.01.2021 17:30

Mathematics, 20.01.2021 17:30

History, 20.01.2021 17:30

History, 20.01.2021 17:30

Mathematics, 20.01.2021 17:30

World Languages, 20.01.2021 17:30


Mathematics, 20.01.2021 17:30

Mathematics, 20.01.2021 17:30

Mathematics, 20.01.2021 17:30

Social Studies, 20.01.2021 17:30

Mathematics, 20.01.2021 17:30

Arts, 20.01.2021 17:30





Mathematics, 20.01.2021 17:30

Mathematics, 20.01.2021 17:30

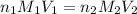
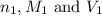 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 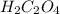
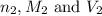 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.