
Chemistry, 27.02.2020 05:57 linreaburg
(a) Compute the radius r of an impurity atom that will just fit into an FCC octahedral site in terms of the atomic radius R of the host atom (without introducing lattice strains). . (b) Repeat part (a) for the FCC tetrahedral site.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
(a) Compute the radius r of an impurity atom that will just fit into an FCC octahedral site in terms...
Questions

Mathematics, 18.11.2020 19:20


Mathematics, 18.11.2020 19:20



Mathematics, 18.11.2020 19:20


English, 18.11.2020 19:20


Chemistry, 18.11.2020 19:20

Chemistry, 18.11.2020 19:20


Mathematics, 18.11.2020 19:20




SAT, 18.11.2020 19:20


Biology, 18.11.2020 19:20


 .
. is as shown in the first uploaded image.
is as shown in the first uploaded image.
 as follows:
as follows:
 as
as and
and  as a and
as a and  as a in above equation as follows:
as a in above equation as follows:

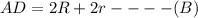


 as a and
as a and  as a in above equation as follows:
as a in above equation as follows:


 for
for 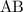 in equation (1) as follows:
in equation (1) as follows: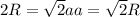
 as follows:
as follows:
 a and
a and  as a in above equation as follows:
as a in above equation as follows:

 in above equation as follows:
in above equation as follows:





