Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
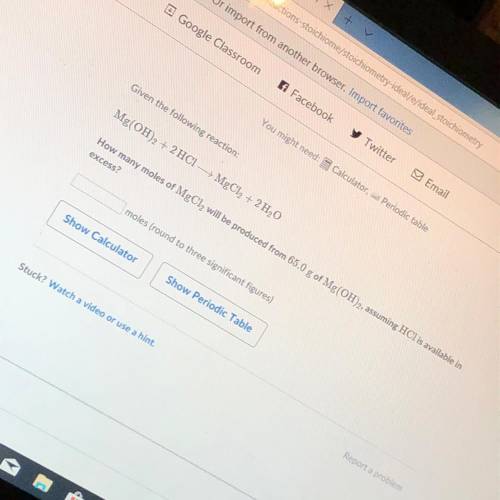
How many moles of MgCl2 will be produced from 65.0 g of Mg(OH)2 assuming HCl is available in excess?...
Questions


Mathematics, 03.11.2020 08:40


Mathematics, 03.11.2020 08:40


Mathematics, 03.11.2020 08:40



English, 03.11.2020 08:40


Mathematics, 03.11.2020 08:40

Health, 03.11.2020 08:40

Chemistry, 03.11.2020 08:40

Mathematics, 03.11.2020 08:40





English, 03.11.2020 08:40