Chemistry, 27.02.2020 09:25 garacey241
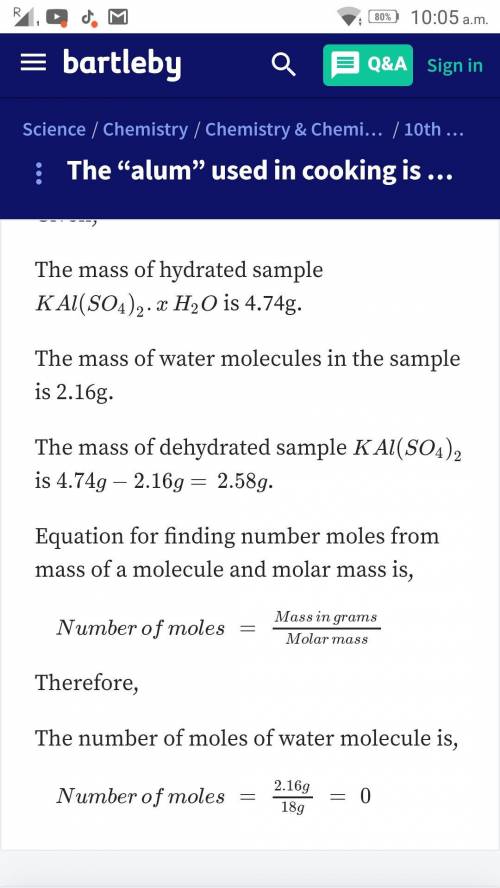
Alum used in cooking is potassium aluminum sulfate hydrate, KAl(SO4)2. XH2O. To find the value of X, you can heat the sample of the compound. Assume you heat 4.74 g of the hydrated compound and that sample loses 2.16 g of water. What is the value of X?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Alum used in cooking is potassium aluminum sulfate hydrate, KAl(SO4)2. XH2O. To find the value of X,...
Questions


English, 09.12.2020 03:10

Mathematics, 09.12.2020 03:10


History, 09.12.2020 03:10

Mathematics, 09.12.2020 03:10

Mathematics, 09.12.2020 03:10

English, 09.12.2020 03:10





Mathematics, 09.12.2020 03:10


Mathematics, 09.12.2020 03:10

Mathematics, 09.12.2020 03:10

Mathematics, 09.12.2020 03:10

Mathematics, 09.12.2020 03:10

Mathematics, 09.12.2020 03:10

English, 09.12.2020 03:10