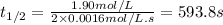The half-life of a reaction, t1/2, is the time required for one-half of a reactant to be consumed. It is the time during which the amount of reactant or its concentration decreases to one-half of its initial value.
Determine the half-life for the reaction in Part B using the integrated rate law, given that the initial concentration is 1.90mol?L?1 and the rate constant is 0.0016mol?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
The half-life of a reaction, t1/2, is the time required for one-half of a reactant to be consumed. I...
Questions

Chemistry, 08.07.2019 00:20

History, 08.07.2019 00:20

Mathematics, 08.07.2019 00:30

History, 08.07.2019 00:30



English, 08.07.2019 00:30


Biology, 08.07.2019 00:30


French, 08.07.2019 00:30

Biology, 08.07.2019 00:30


English, 08.07.2019 00:30

Business, 08.07.2019 00:30





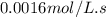
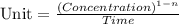
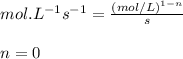
![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0526/7626/b5b11.png)
![[A_o]](/tpl/images/0526/7626/dc622.png) = initial concentration = 1.90 mol/L
= initial concentration = 1.90 mol/L