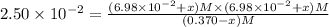Chemistry, 27.02.2020 20:13 SKYBLUE1015
Some SbCl5 is allowed to dissociate into SbCl3 and Cl2 at 521 K. At equilibrium, [SbCl5] = 0.195 M, and [SbCl3] = [Cl2] = 6.98×10-2 M. Additional SbCl5 is added so that [SbCl5]new = 0.370 M and the system is allowed to once again reach equilibrium.
SbCl5(g) <-> SbCl3(g) + Cl2(g) K = 2.50×10-2 at 521 K
(a) In which direction will the reaction proceed to reach equilibrium? to the right to the left
(b) What are the new concentrations of reactants and products after the system reaches equilibrium?
[SbCl5] = M
[SbCl3] = ___M
[Cl2] = ___M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

You know the right answer?
Some SbCl5 is allowed to dissociate into SbCl3 and Cl2 at 521 K. At equilibrium, [SbCl5] = 0.195 M,...
Questions

Mathematics, 13.01.2021 01:00


Mathematics, 13.01.2021 01:00


Biology, 13.01.2021 01:00


Spanish, 13.01.2021 01:00


Health, 13.01.2021 01:00



English, 13.01.2021 01:00


Mathematics, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00

Arts, 13.01.2021 01:00


Biology, 13.01.2021 01:00


![[SbCl_5]=(0.370-x) M=(0.370-0.0233) M=0.3467 M](/tpl/images/0527/0501/9b64f.png)
![[SbCl_3]=(6.98\times 10^{-2}+x) M=(6.98\times 10^{-2}+0.0233) M=0.0931 M](/tpl/images/0527/0501/4f238.png)
![[Cl_2]=(6.98\times 10^{-2}+x) M=(6.98\times 10^{-2}+0.0233) M=0.0931 M](/tpl/images/0527/0501/5868d.png)
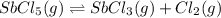
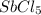 is increasing .So, the equilibrium will shift in the right direction.
is increasing .So, the equilibrium will shift in the right direction.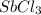 =
= 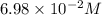
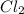 =
= 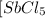 to 0.370 M at equilibrium :
to 0.370 M at equilibrium :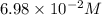
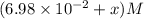
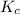
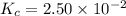
![K_c=\frac{[SbCl_3][Cl_2]}{[SbCl_5]}](/tpl/images/0527/0501/c5c78.png)