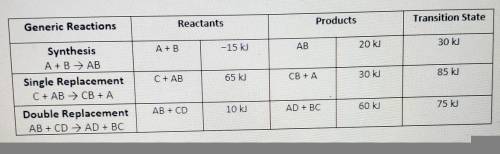
(View table above)
To assist you, use the enthalpy values in the data chart for each gene...

(View table above)
To assist you, use the enthalpy values in the data chart for each generic reaction provided. Be sure to following the summary of steps below.
• Illustrate the x- and y-axes to show the reaction pathway and potential energy, in kilojoules. Ensure your energy intervals are appropriate for the data
• Plot the enthalpy values of the reactants, products, and transition state using three horizontal dotted lines across the graph for each
• Draw the energy curve from the reactants line to the transition state and curve the line back down to the energy of the products. Label the reactants, products, and transition state.
• Illustrate double-headed arrows to represent both the total change in enthalpy (ΔH) and the activation energy (Ea).
• Calculate the total change in enthalpy and the activation energy using the energy values provided for each reaction. Record those values below the graph.
• Make sure correct units are included.
Conclusion Statement
Write a two to four sentence conclusion statement explaining how the potential energy diagram is used to identify if the reaction is endothermic or exothermic, if heat was released or absorbed, and why the sign of enthalpy change was positive of negative. There should be a conclusion statement for each graph.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Questions

Arts, 28.01.2021 02:20


Chemistry, 28.01.2021 02:20


Mathematics, 28.01.2021 02:20

History, 28.01.2021 02:20


Mathematics, 28.01.2021 02:20


Mathematics, 28.01.2021 02:20


History, 28.01.2021 02:20

Mathematics, 28.01.2021 02:20

English, 28.01.2021 02:20


Mathematics, 28.01.2021 02:20

Mathematics, 28.01.2021 02:20


Mathematics, 28.01.2021 02:20



