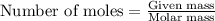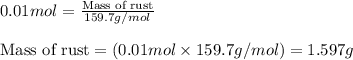Oxalic acid can remove rust (Fe2O3) caused by bathtub rings according to the reaction Fe2O3(s) - 6H2C2O4(aq) rightarrow 2Fe(C2O4)33-(aq) - 3H2O(l) - 6H+(aq) C calculate the number of grams of rust that can be removed by 6.00 times 102 mL of a 0.100 M solution of oxalic acid.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
Oxalic acid can remove rust (Fe2O3) caused by bathtub rings according to the reaction Fe2O3(s) - 6H2...
Questions

Computers and Technology, 04.03.2021 18:00






Chemistry, 04.03.2021 18:00



Mathematics, 04.03.2021 18:00

History, 04.03.2021 18:00


Mathematics, 04.03.2021 18:00

Social Studies, 04.03.2021 18:00


History, 04.03.2021 18:00

Mathematics, 04.03.2021 18:00


Health, 04.03.2021 18:00

World Languages, 04.03.2021 18:00

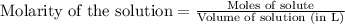 .....(1)
.....(1)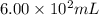 = 600 mL = 0.600 L (Conversion factor: 1 L = 1000 mL)
= 600 mL = 0.600 L (Conversion factor: 1 L = 1000 mL)
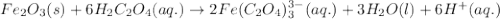
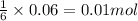 of ferric oxide (rust)
of ferric oxide (rust)