
Chemistry, 27.02.2020 22:19 lizzytyler4725
A 10.000g ice cube is added to 100.000g of water at 80.0 oC in a calorimeter. The final temperature of the water in the calorimeter is 65.5 oC. How much heat in kilojoules was needed to melt the ice cube?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
A 10.000g ice cube is added to 100.000g of water at 80.0 oC in a calorimeter. The final temperature...
Questions



History, 13.10.2020 03:01

Geography, 13.10.2020 03:01







Computers and Technology, 13.10.2020 03:01






Computers and Technology, 13.10.2020 03:01



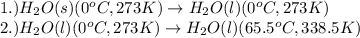
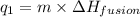
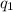 = amount of heat absorbed = ?
= amount of heat absorbed = ?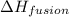 = enthalpy change for fusion = 334.16 J/g
= enthalpy change for fusion = 334.16 J/g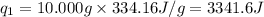
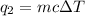
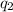 = heat absorbed
= heat absorbed = change in temperature =
= change in temperature = ![T_2-T_1=[65.0-0]^oC=65.5^oC](/tpl/images/0527/2479/63e8e.png)
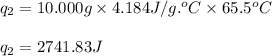
![[3341.6+2741.83]J=6083.43J=6.083kJ](/tpl/images/0527/2479/8ebc5.png)


