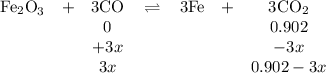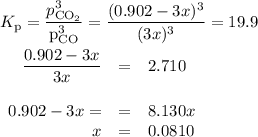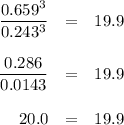Chemistry, 27.02.2020 22:41 bartekpiglo
At 1000 K, Kp=19.9 for the reaction Fe2O3(s)+3CO(g)<--->2Fe(s)+3C O2(g) What are the equilibrium partial pressures of CO and if CO2 is the only gas present initially, at a partial pressure of 0.902 ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
At 1000 K, Kp=19.9 for the reaction Fe2O3(s)+3CO(g)<--->2Fe(s)+3C O2(g) What are the equilibri...
Questions



Mathematics, 05.05.2020 20:31



Health, 05.05.2020 20:31


Computers and Technology, 05.05.2020 20:31


Mathematics, 05.05.2020 20:31