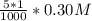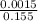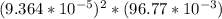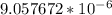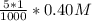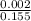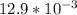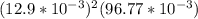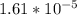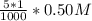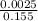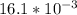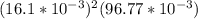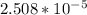For which of the following mixtures will Ag2SO4(s) precipitate?
a. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.20 M AgNO3(aq)
b. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.30 M AgNO3(aq)
c. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.40 M AgNO3(aq)
d. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.50 M AgNO3(aq)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
For which of the following mixtures will Ag2SO4(s) precipitate?
a. 150.0 mL of 0.10 M Na2SO4(a...
a. 150.0 mL of 0.10 M Na2SO4(a...
Questions

Social Studies, 24.09.2019 15:20


Arts, 24.09.2019 15:20



History, 24.09.2019 15:20

Mathematics, 24.09.2019 15:20


History, 24.09.2019 15:20


Computers and Technology, 24.09.2019 15:20

Mathematics, 24.09.2019 15:20




Geography, 24.09.2019 15:20




English, 24.09.2019 15:20

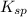 =
= ![[Ag^+]^2[SO_4^{2-}]](/tpl/images/0527/4149/191c0.png)
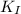 <
< 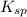 ; precipitation will not occur
; precipitation will not occur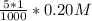
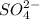 =
= 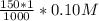
![[Ag^+]^2](/tpl/images/0527/4149/1aebd.png) =
= 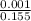

![[SO_4^{2-}]](/tpl/images/0527/4149/43b69.png) =
= 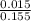

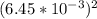 ×
×