
Chemistry, 28.02.2020 02:23 melissapulido198
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-2 at 566 K .The reaction is allowed to reach equilibrium in a 7.40-L flask. At equilibrium, [SbCl5] = 0.333 M, [SbCl3] = 8.75×10-2 M and [Cl2] = 8.75×10-2 M.
(a) The equilibrium mixture is transferred to a 14.8-L flask. In which direction will the reaction proceed to reach equilibrium?
(b) Calculate the new equilibrium concentrations that result when the equilibrium mixture is transferred to a 14.8-L flask.
[SbCl5] = M
[SbCl3] = M
[Cl2] = M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1


Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-...
Questions



Computers and Technology, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00

Computers and Technology, 18.01.2021 14:00




Computers and Technology, 18.01.2021 14:00



Mathematics, 18.01.2021 14:00




Computers and Technology, 18.01.2021 14:00



Biology, 18.01.2021 14:00

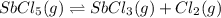
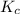
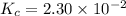
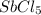 in 7.40 L = 0.333 M
in 7.40 L = 0.333 M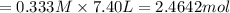
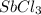 in 7.40 L =
in 7.40 L = 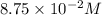
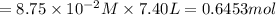
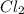 in 7.40 L =
in 7.40 L = 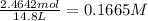
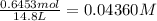
![K_c=\frac{[SbCl_3][Cl_2]}{[SbCl_5]}](/tpl/images/0527/6048/c5c78.png)
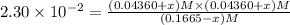
![[SbCl_5]=(0.1665-x) M=(0.1665-0.01536) M=0.1511 M](/tpl/images/0527/6048/35e3f.png)
![[SbCl_3]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/a8c15.png)
![[Cl_2]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/dab14.png)


