
Chemistry, 28.02.2020 03:16 makayla7635
A 3.0 L flask containing helium at 145 mmHg is connected by a closed valve to a 2.0 L flask containing argon at 355 mmHg. When the valve is opened and the gases are allowed to mix equally in the two flasks, what is the total pressure (in mmHg) in the two connected flasks after mixing ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
A 3.0 L flask containing helium at 145 mmHg is connected by a closed valve to a 2.0 L flask containi...
Questions



History, 30.09.2020 04:01



Mathematics, 30.09.2020 04:01


History, 30.09.2020 04:01

History, 30.09.2020 04:01


Chemistry, 30.09.2020 04:01




Biology, 30.09.2020 04:01





Mathematics, 30.09.2020 04:01

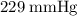 .
. 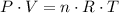 , where
, where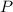 is the pressure of the sample.
is the pressure of the sample.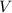 is the volume of the container.
is the volume of the container.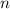 is the number of moles of gas particles in the sample.
is the number of moles of gas particles in the sample.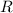 is the ideal gas constant.
is the ideal gas constant.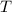 is the temperature of the sample.
is the temperature of the sample.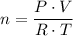 , and
, and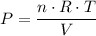 .
.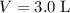 and
and 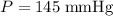 . Hence,
. Hence, 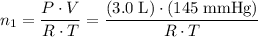 .In the argon container,
.In the argon container, 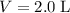 and
and 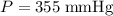 . Hence,
. Hence, 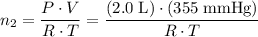 .
.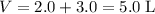 . Assuming that temperature
. Assuming that temperature 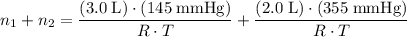 .
. .
.

