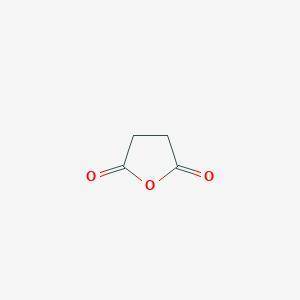
Chemistry, 28.02.2020 04:38 shelbylynn17
A compound A has prominent infrared absorptions at 1050, 1786, and 1852 cm–1 and shows a single absorption in the proton NMR spectrum at δ 3.00. When heated gently with methanol, compound B, C5H8O4, is obtained. Compound B has IR absorptions at 2500–3000 (broad), 1730, and 1701 cm–1, and its proton NMR spectrum in D2O consists of resonances at δ 2.7 (complex splitting) and δ 3.7 (a singlet) in the intensity ratio 4:3. Give the structures A and B, omitting stereochemistry.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
A compound A has prominent infrared absorptions at 1050, 1786, and 1852 cm–1 and shows a single abso...
Questions

Geography, 13.11.2020 20:10

Mathematics, 13.11.2020 20:10

Biology, 13.11.2020 20:10


Mathematics, 13.11.2020 20:10

Mathematics, 13.11.2020 20:10


Biology, 13.11.2020 20:10

History, 13.11.2020 20:10

Mathematics, 13.11.2020 20:10


Advanced Placement (AP), 13.11.2020 20:10


Arts, 13.11.2020 20:10


Mathematics, 13.11.2020 20:10

Chemistry, 13.11.2020 20:10

World Languages, 13.11.2020 20:10

History, 13.11.2020 20:10

Health, 13.11.2020 20:10