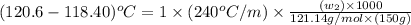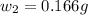A certain liquid X has a normal boiling point of 118.40 C and a boiling point elevation constant K,-240 С kg-mol-1. A solution is prepared by dissolving some benzamide (C, H,NO) in 150. g of X. This solution boils at 120.6 C. Calculate the mass of C, H,NO that was dissolved Be sure your answer is rounded to the correct number of significiant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
A certain liquid X has a normal boiling point of 118.40 C and a boiling point elevation constant K,-...
Questions


Mathematics, 02.11.2019 20:31

Mathematics, 02.11.2019 20:31

History, 02.11.2019 20:31



Spanish, 02.11.2019 20:31


Mathematics, 02.11.2019 20:31



History, 02.11.2019 20:31


Mathematics, 02.11.2019 20:31

Mathematics, 02.11.2019 20:31

Mathematics, 02.11.2019 20:31

Computers and Technology, 02.11.2019 20:31

Mathematics, 02.11.2019 20:31

Arts, 02.11.2019 20:31

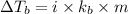
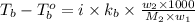
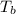 = boiling point of solution =
= boiling point of solution = 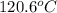
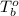 = boiling point of liquid X =
= boiling point of liquid X = 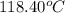
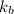 = boiling point constant of liquid X =
= boiling point constant of liquid X = 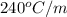
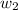 = mass of solute (benzamide ) = ?
= mass of solute (benzamide ) = ?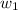 = mass of solvent (liquid X) = 150 g
= mass of solvent (liquid X) = 150 g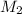 = molar mass of solute (benzamide ) = 121.14 g/mol
= molar mass of solute (benzamide ) = 121.14 g/mol