
Chemistry, 28.02.2020 18:56 kobiemajak
The value for Kw is 1.0 x 10-14 at 298K, but this value is temperature dependent. Given what you know about Kw and acid/base chemistry, what will the pH of water be at 283K if the value for Kw is 0.29 x 10-14? Is this solution acidic, basic, or neutral?
a. pH = 7.26, acidic
b. pH = 7.26, neutral
c. pH = 7.00, neutral
d. pH = 14.54, basic
e. pH = 7.26, basic

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
The value for Kw is 1.0 x 10-14 at 298K, but this value is temperature dependent. Given what you kno...
Questions





Health, 08.01.2020 07:31


Health, 08.01.2020 07:31











Biology, 08.01.2020 07:31


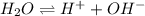
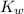
![K_w=[H^+][OH^-]](/tpl/images/0528/0368/bc68a.png)
![K_w=[H^+][H^+]=[H^+]^2](/tpl/images/0528/0368/7edc9.png)
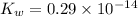
![K_w=[H^+]^2](/tpl/images/0528/0368/c7d3c.png)
![0.29\times 10^{-14}=[H^+]^2](/tpl/images/0528/0368/14418.png)
![[H^+]=5.385\times 10^{-8} M](/tpl/images/0528/0368/0b02c.png)
![pH=-\log [H^+]](/tpl/images/0528/0368/37e81.png)
![=-\log[5.385\times 10^{-8} M]=7.26](/tpl/images/0528/0368/66da4.png)


