
Chemistry, 28.02.2020 18:59 moomoo2233
Write out the following statements in balanced chemical reactions. Specify states of matter for each substance. Watch out for elements that exist as diatomic molecules! a) Sodium reacts with sulfur to form sodium sulfide. (The most common form of sulfur is S8(s) so that is what is expected to be used here).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Write out the following statements in balanced chemical reactions. Specify states of matter for each...
Questions

Biology, 30.06.2021 14:00

History, 30.06.2021 14:00



Physics, 30.06.2021 14:00



Biology, 30.06.2021 14:00



Geography, 30.06.2021 14:00



English, 30.06.2021 14:00

SAT, 30.06.2021 14:00

Computers and Technology, 30.06.2021 14:00


Mathematics, 30.06.2021 14:00


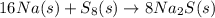
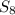 to give 8 moles of
to give 8 moles of 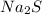 .
.


