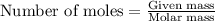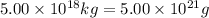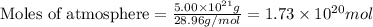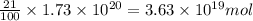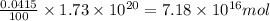The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 are present in the atmosphere? Note that it is important to work in units of moles rather than in units of mass. By the ideal gas law, PV=nRT. P is pressure, V is volume, n is the number of moles, T is temperature (K), and R is the gas constant. At a given temperature and pressure, the volume is proportional to the number of moles, not to the mass.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 ar...
Questions

Mathematics, 08.04.2020 21:39



English, 08.04.2020 21:39

English, 08.04.2020 21:39

Mathematics, 08.04.2020 21:39



Computers and Technology, 08.04.2020 21:39

Mathematics, 08.04.2020 21:39

History, 08.04.2020 21:39



Mathematics, 08.04.2020 21:39


Mathematics, 08.04.2020 21:39



Biology, 08.04.2020 21:39

English, 08.04.2020 21:39

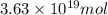 and
and 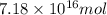 respectively
respectively