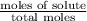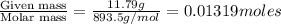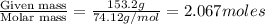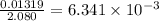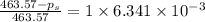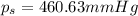Chemistry, 28.02.2020 19:30 samantha636
The vapor pressure of diethyl ether (ether) is 463.57 mm Hg at 25 °C. A nonvolatile, nonelectrolyte that dissolves in diethyl ether is chlorophyll. Calculate the vapor pressure of the solution at 25 °C when 11.79 grams of chlorophyll, C55H72MgN4O5 (893.5 g/mol), are dissolved in 153.2 grams of diethyl ether. diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol. VP(solution) = mm Hg

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
The vapor pressure of diethyl ether (ether) is 463.57 mm Hg at 25 °C. A nonvolatile, nonelectrolyte...
Questions

Mathematics, 16.07.2019 15:30




History, 16.07.2019 15:30

History, 16.07.2019 15:30

History, 16.07.2019 15:30


Biology, 16.07.2019 15:30

Mathematics, 16.07.2019 15:30


History, 16.07.2019 15:30

Biology, 16.07.2019 15:30

Biology, 16.07.2019 15:30


History, 16.07.2019 15:30

Mathematics, 16.07.2019 15:30



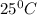 is 460.63 mmHg
is 460.63 mmHg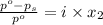
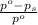 = relative lowering in vapor pressure
= relative lowering in vapor pressure
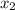 = mole fraction of solute =
= mole fraction of solute =