
Chemistry, 28.02.2020 19:28 ballerboles4747
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The half-life of phosphorus-32 is 14.3 days. If you begin with 30.5 mg of this isotope, what mass remains after 27.5 days have passed? Since the decomposition is a radioactive decay reaction, it is first order.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2


Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The hal...
Questions

Mathematics, 13.05.2021 23:50

Mathematics, 13.05.2021 23:50

Mathematics, 13.05.2021 23:50

Mathematics, 13.05.2021 23:50




Mathematics, 13.05.2021 23:50



Mathematics, 13.05.2021 23:50



Mathematics, 13.05.2021 23:50





Mathematics, 13.05.2021 23:50

Mathematics, 13.05.2021 23:50

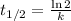
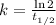
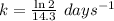
![[A_t]=[A_0]e^{-kt}](/tpl/images/0528/1963/1ef89.png)
![[A_t]](/tpl/images/0528/1963/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0528/1963/9a686.png) is the initial concentration = 30.5 mg
is the initial concentration = 30.5 mg![[A_t]=30.5\times e^{-0.04847\times 27.5}\ mg=8.043\ mg](/tpl/images/0528/1963/500e7.png)


