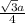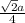Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

You know the right answer?
Solid vanadium crystallizes in a body-centered cubic structure and has a density of 6.00 g/cm3. Firs...
Questions

Mathematics, 07.05.2021 01:00



English, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00






History, 07.05.2021 01:00


English, 07.05.2021 01:00


Arts, 07.05.2021 01:00



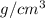
![\sqrt[3]{\frac{mass}{density \times N_{A}}}](/tpl/images/0528/4230/02dd5.png)
![\sqrt[3]{\frac{50.941 g/mol}{6.0 g/cm^{3} \times 6.022 \times 10^{23}}}](/tpl/images/0528/4230/29210.png)
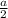
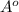 which is not right.
which is not right.