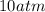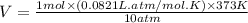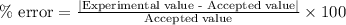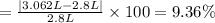Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
One gram-mole of methyl chloride vapor is contained in a vessel at 100°C and 10atm.(a) Use the ideal...
Questions

English, 04.06.2021 18:30

Mathematics, 04.06.2021 18:30

Mathematics, 04.06.2021 18:30

Mathematics, 04.06.2021 18:30

Mathematics, 04.06.2021 18:30


Mathematics, 04.06.2021 18:30


History, 04.06.2021 18:30




Mathematics, 04.06.2021 18:30

Mathematics, 04.06.2021 18:30

Social Studies, 04.06.2021 18:30


History, 04.06.2021 18:30

Physics, 04.06.2021 18:30


Mathematics, 04.06.2021 18:30