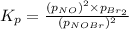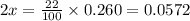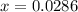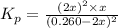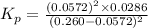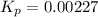Chemistry, 28.02.2020 21:57 alexismurcia550
Nitrosyl bromide decomposes according to the chemical equation below. 2NOBr(g) ↔ 2NO(g) + Br2(g) When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium, 22% of the NOBr decomposes. What is the equilibrium constant, Kp, for the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1


Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
Nitrosyl bromide decomposes according to the chemical equation below. 2NOBr(g) ↔ 2NO(g) + Br2(g) Whe...
Questions


Mathematics, 14.10.2019 15:50

History, 14.10.2019 15:50


English, 14.10.2019 15:50


Mathematics, 14.10.2019 15:50


Mathematics, 14.10.2019 16:00

Mathematics, 14.10.2019 16:00


History, 14.10.2019 16:00


Mathematics, 14.10.2019 16:00


Mathematics, 14.10.2019 16:00




Mathematics, 14.10.2019 16:00

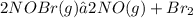 "
" 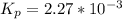
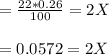
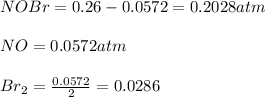
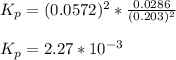
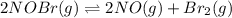
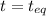 0.260-2x 2x x
0.260-2x 2x x
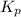 for the given reaction follows:
for the given reaction follows: