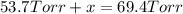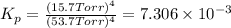Chemistry, 28.02.2020 22:46 AutumnJoy12
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressure of water vapor is 53.7 torr, the total pressure at equilibrium is 69.4 torr. Calculate Kp for this reaction at 1181 K. 3 Fe(s) + 4 H2O(g) equilibrium reaction arrow Fe3O4(s) + 4 H2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressu...
Questions






Mathematics, 29.07.2019 19:00






Mathematics, 29.07.2019 19:00


English, 29.07.2019 19:00

English, 29.07.2019 19:00

History, 29.07.2019 19:00




Spanish, 29.07.2019 19:00

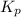 for this reaction at 1181 K is
for this reaction at 1181 K is  .
.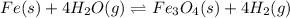
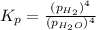
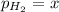
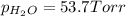
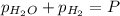 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)