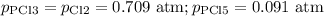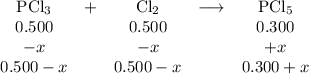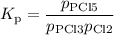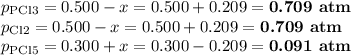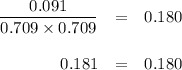Chemistry, 29.02.2020 00:17 Dogtes9667
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.
A flask is charged with 0.500 atm PCl3 , 0.500 atm Cl2, and 0.300atm PCl5 at this temperature.
What are the equilibrium partial pressures of PCl3 , Cl2, and PCl5, respectively?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
Questions

Mathematics, 08.01.2020 10:31



Chemistry, 08.01.2020 10:31


Mathematics, 08.01.2020 10:31


Mathematics, 08.01.2020 10:31






Computers and Technology, 08.01.2020 10:31

Mathematics, 08.01.2020 10:31

Chemistry, 08.01.2020 10:31

English, 08.01.2020 10:31