
Chemistry, 28.02.2020 23:27 emobirdy1122
Consider the following multistep reaction: A+B→AB(slow) A+AB→A2B(fast)–––––––––––––––––––– 2A+B→A2B(overall) Based on this mechanism, determine the rate law for the overall reaction. Express your answer in standard MasteringChemistry format. For example, if the rate law is k[A]3[B]2 type k*[A]^3*[B]^2. View Available Hint(s)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
Consider the following multistep reaction: A+B→AB(slow) A+AB→A2B(fast)–––––––––––––––––––– 2A+B→A2B(...
Questions



Mathematics, 05.09.2020 19:01




English, 05.09.2020 19:01


Physics, 05.09.2020 19:01



Chemistry, 05.09.2020 19:01






Advanced Placement (AP), 05.09.2020 19:01

Mathematics, 05.09.2020 19:01

![Rate=k[A][B]](/tpl/images/0528/6935/27e48.png)
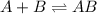 (slow)
(slow)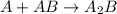 (fast)
(fast)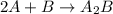 (overall)
(overall)

