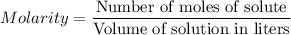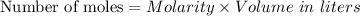Chemistry, 29.02.2020 01:25 samanthacruzsc51
For each trial, compute the mol of titrant; (molarity x L) and keep the number of significant figures to 4.
Trial 1: 12.49 mL =
mol NaOH
Trial 2: 12.32 mL =
mol NaOH
Trial 3: 11.87 mL =
mol NaOH

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3



Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
For each trial, compute the mol of titrant; (molarity x L) and keep the number of significant figure...
Questions


Mathematics, 21.02.2022 22:50

Mathematics, 21.02.2022 22:50

Spanish, 21.02.2022 22:50

Mathematics, 21.02.2022 22:50

Mathematics, 21.02.2022 22:50


Health, 21.02.2022 23:00



Mathematics, 21.02.2022 23:00

Mathematics, 21.02.2022 23:00




Mathematics, 21.02.2022 23:00



Mathematics, 21.02.2022 23:10