
Chemistry, 29.02.2020 01:51 heyysiirr3064
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily increases, but that of O is less than that of N. The best interpretation of the lowervalue for O is that:1. the ionization potential of N is a maximum and the values decrease steadily for the elements O, F, and Ne.2. there is more shielding of the nuclear charge in O than in B, C, or N.3. the electron removed from O is farther from the nucleus and therefore less tightly bound than that in N.4. the electron removed from O corresponds to a different value of the quantum number L than that of the electron removed from B, C, or N.5. the half-filled set of p orbitals in N makes it more difficult to remove an electron from N than from O.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily inc...
Questions

Mathematics, 27.05.2021 17:40

Mathematics, 27.05.2021 17:40



Biology, 27.05.2021 17:40


Mathematics, 27.05.2021 17:40

Mathematics, 27.05.2021 17:40

Mathematics, 27.05.2021 17:40


History, 27.05.2021 17:40




Mathematics, 27.05.2021 17:40

English, 27.05.2021 17:40


Mathematics, 27.05.2021 17:40


Mathematics, 27.05.2021 17:40

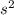 2
2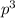 (half-filled orbital), Which allows it additional stability.
(half-filled orbital), Which allows it additional stability.

