
Antimony pentachloride decomposes according to this equation: SbCl5(g)⇌SbCl3(g)+Cl2(g) An equilibrium mixture in a 5.00-L flask at 448 °C contains 3.85 g of SbCl5, 9.14 g of SbCl3, and 2.84 g of Cl2. How many grams of each will be found if the mixture is transferred into a 2.00-L flask at the same temperature?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2


Chemistry, 23.06.2019 22:30
The barometer shown in fig. p1.35 contains mercury ( 5 13.59 g/cm3 ). if the local atmospheric pressure is 100 kpa and g 5 9.81 m/s2 , determine the height of the mercury column, l, in mmhg and inhg.
Answers: 1

Chemistry, 24.06.2019 07:00
Question 14 fill in the left side of this equilibrium constant equation for the reaction of acetic acid hch3co2 with water.
Answers: 1
You know the right answer?
Antimony pentachloride decomposes according to this equation: SbCl5(g)⇌SbCl3(g)+Cl2(g) An equilibriu...
Questions

Health, 30.01.2020 05:55




English, 30.01.2020 05:55


Mathematics, 30.01.2020 05:55


History, 30.01.2020 05:56

Biology, 30.01.2020 05:56

Mathematics, 30.01.2020 05:56


Biology, 30.01.2020 05:56

Mathematics, 30.01.2020 05:56


Mathematics, 30.01.2020 05:56

History, 30.01.2020 05:56

English, 30.01.2020 05:56

Business, 30.01.2020 05:56

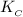 =
= ![\frac{[SbCl_3][Cl_2]}{[SbCl_5]}](/tpl/images/0528/9275/e005c.png)
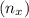 =
= 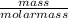
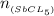 =
= 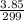
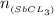 =
= 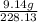
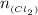 =
= 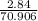
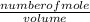
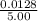
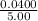
![\frac{[8*10^{-3}][8*10^{-3}]}{[2.56*10^{-3}]}](/tpl/images/0528/9275/199f0.png)
![\frac{[y][y]}{[y]}](/tpl/images/0528/9275/d2bce.png)


