
Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron acceptor. NO2- 6e- -> NH4 (-0.41 volts) O2 4e- -> 2H2O ( 0.82 volts) If you balance and combine the reactions so that 28 molecules of NH4 are oxidized to NO2-, how many molecules of O2 will be reduced to H2O

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2


Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a s...
Questions

English, 15.12.2020 02:10


Mathematics, 15.12.2020 02:10

Mathematics, 15.12.2020 02:10






English, 15.12.2020 02:10

Chemistry, 15.12.2020 02:10

Biology, 15.12.2020 02:10



English, 15.12.2020 02:10

Mathematics, 15.12.2020 02:10

Mathematics, 15.12.2020 02:10

Mathematics, 15.12.2020 02:10


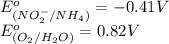
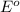 potential will always get reduced and will undergo reduction reaction. Here, oxygen will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, oxygen will undergo reduction reaction will get reduced.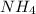 will undergo oxidation reaction and will get oxidized.
will undergo oxidation reaction and will get oxidized.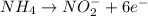 ( × 4)
( × 4)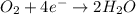 ( × 6)
( × 6)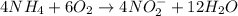
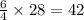 molecules of oxygen gas
molecules of oxygen gas

