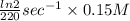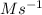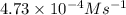Chemistry, 29.02.2020 03:23 tobywaffle1234
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces a plot of Ln[(CH3)3CCl] vs. time that is linear with a negative slope. Suppose the reaction is carried out under conditions such that the half-life of the reaction is 2.20 x 102 s. What is the instantaneous rate of reaction when [(CH3)3CCl] = 0.15 M?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces...
Questions

Arts, 27.09.2019 08:30


History, 27.09.2019 08:30

Chemistry, 27.09.2019 08:30

Mathematics, 27.09.2019 08:30




History, 27.09.2019 08:30

Mathematics, 27.09.2019 08:30

Mathematics, 27.09.2019 08:30


English, 27.09.2019 08:30


Mathematics, 27.09.2019 08:30

History, 27.09.2019 08:30



Mathematics, 27.09.2019 08:30

Biology, 27.09.2019 08:30

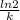
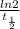
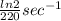
![[(CH_{3})_{3}CCl]^{1}](/tpl/images/0528/9812/8a6e5.png)