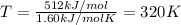Chemistry, 29.02.2020 04:18 Mistytrotter
As an approximation, we can assume that proteins exist either in their native (physiologically relevant) state or in a denatured state. The standard molar enthalpy and entropy of denaturationof a certain protein are 512 kJ/mol and 1.60 kJ/(mol K), respectively. Assume that DHand DSare independent of temperature. a. Commenton the signs thinking about protein denaturation. b. Calculate the standard molar free energy of denaturation. c. Calculate the temperature at which the denaturation becomes spontaneous.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
As an approximation, we can assume that proteins exist either in their native (physiologically relev...
Questions



Mathematics, 14.12.2019 07:31


Mathematics, 14.12.2019 07:31

Chemistry, 14.12.2019 07:31

Mathematics, 14.12.2019 07:31



Mathematics, 14.12.2019 07:31


Social Studies, 14.12.2019 07:31


Social Studies, 14.12.2019 07:31



Arts, 14.12.2019 07:31

Health, 14.12.2019 07:31