
Chemistry, 29.02.2020 04:26 sierra6816
A flask is filled with 6.0 atm of N2 and 6.0 atm of H2. The gases react and NH3 is formed. What is the pressure in the flask after the reaction occurs as completely as possible

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
A flask is filled with 6.0 atm of N2 and 6.0 atm of H2. The gases react and NH3 is formed. What is t...
Questions

History, 25.09.2019 15:30

Chemistry, 25.09.2019 15:30

Mathematics, 25.09.2019 15:30


English, 25.09.2019 15:30



Physics, 25.09.2019 15:30



English, 25.09.2019 15:30







English, 25.09.2019 15:30

History, 25.09.2019 15:30

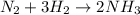
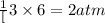 of nitrogen gas
of nitrogen gas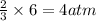 of ammonia
of ammonia


