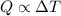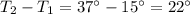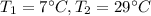Chemistry, 29.02.2020 05:25 kellimcollier8294
A container with a temperature T1 of 15°C is submerged in a bucket of hot water with a temperature T2 of 37°C. An identical container with a temperature of T1 is submerged in an identical bucket of water with a temperature of T2. If the amounts of energy transferred as heat between the containers and the water are the same in both cases, which of the following statements is true?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
A container with a temperature T1 of 15°C is submerged in a bucket of hot water with a temperature T...
Questions

Mathematics, 05.01.2020 22:31

SAT, 05.01.2020 22:31

Mathematics, 05.01.2020 22:31






Mathematics, 05.01.2020 22:31





Mathematics, 05.01.2020 22:31




Mathematics, 05.01.2020 22:31


Mathematics, 05.01.2020 22:31